Current Research Projects-General
Description
Our general research
interests are in the area of biological chemistry. Currently, we are engaged
in three projects. One of these is aimed at the discovery of the mechanism(s)
of directed protein evolution in microbes. By learning how genes are recruited
and retooled for adaptation to sudden influxes of toxins or unnatural organics,
strategies for inhibiting the acquisition of drug resistance should follow.
In addition, we hope to uncover general strategies which can be used to
engineer new chemical pathways in microorganisms for application in relay
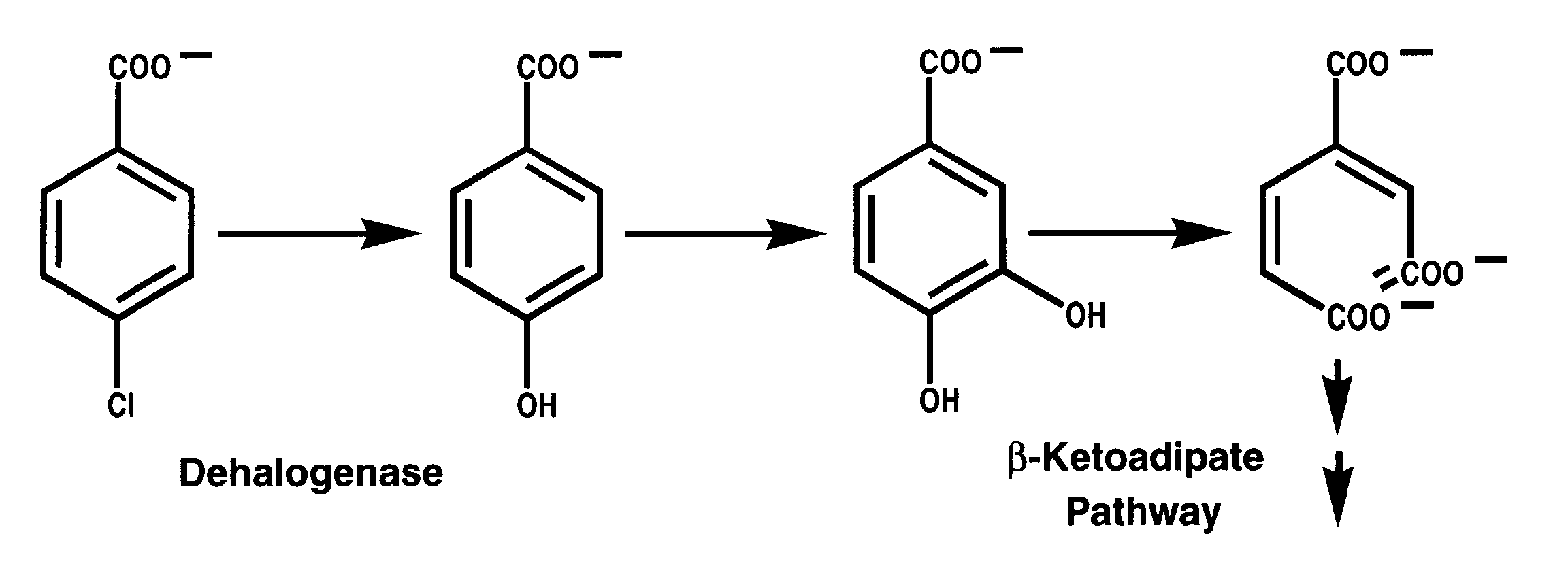
synthesis and bioremediation. At the moment , studies are focused on the
elucidation of the chemistry and genetics of the 4-chlorobenzoate degrading
pathway recruited by soil bacterial residents of polychlorinated biphenyl
(PCBs) spill sites.
 A second area of investigation
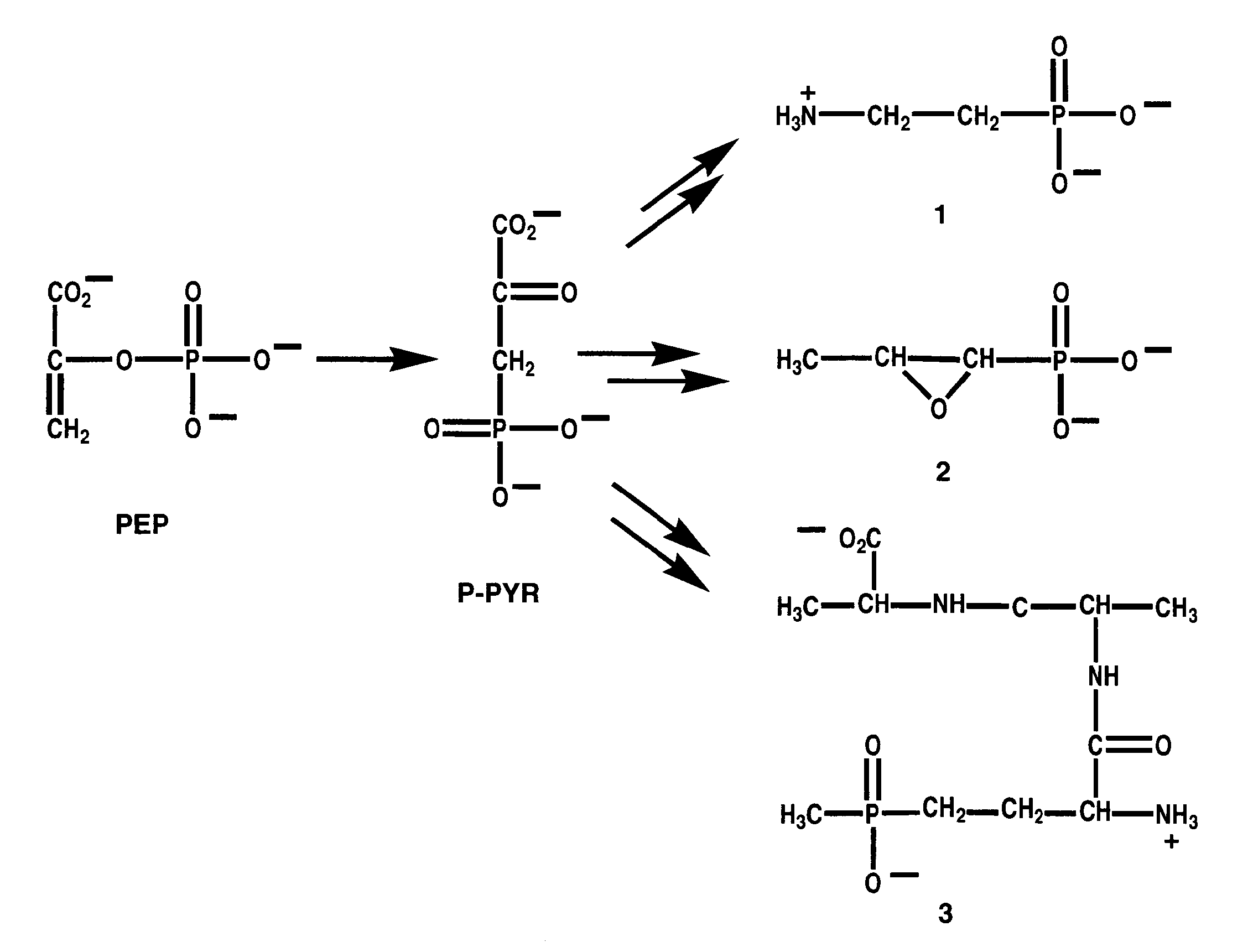
is concerned with the biochemistry of natural and synthetic phosphonates.
Phosphonates belong to a growing family of biologically active organophosphorus
compounds containing P-C bonds. Phosphonates are chemically stable phosphate
ester analogs and, as such, find widespread use as herbicides, pesticides
and pharmaceutical agents. Natural phosphonates are diverse both in structure
and occurrence. In certain strains of bacteria, they are produced as secondary
metabolites having antibiotic activity. Their physiological functions in
higher organisms are largely unknown but evidence which links them to cell
signaling processes has been reported. Studies are presently being conducted
to define the biosynthetic and biodegradative pathways of phosphonates
and the mechanisms of the enzymes catalyzing P-C formation and cleavage.
A second area of investigation
is concerned with the biochemistry of natural and synthetic phosphonates.
Phosphonates belong to a growing family of biologically active organophosphorus
compounds containing P-C bonds. Phosphonates are chemically stable phosphate
ester analogs and, as such, find widespread use as herbicides, pesticides
and pharmaceutical agents. Natural phosphonates are diverse both in structure
and occurrence. In certain strains of bacteria, they are produced as secondary
metabolites having antibiotic activity. Their physiological functions in
higher organisms are largely unknown but evidence which links them to cell
signaling processes has been reported. Studies are presently being conducted
to define the biosynthetic and biodegradative pathways of phosphonates
and the mechanisms of the enzymes catalyzing P-C formation and cleavage.
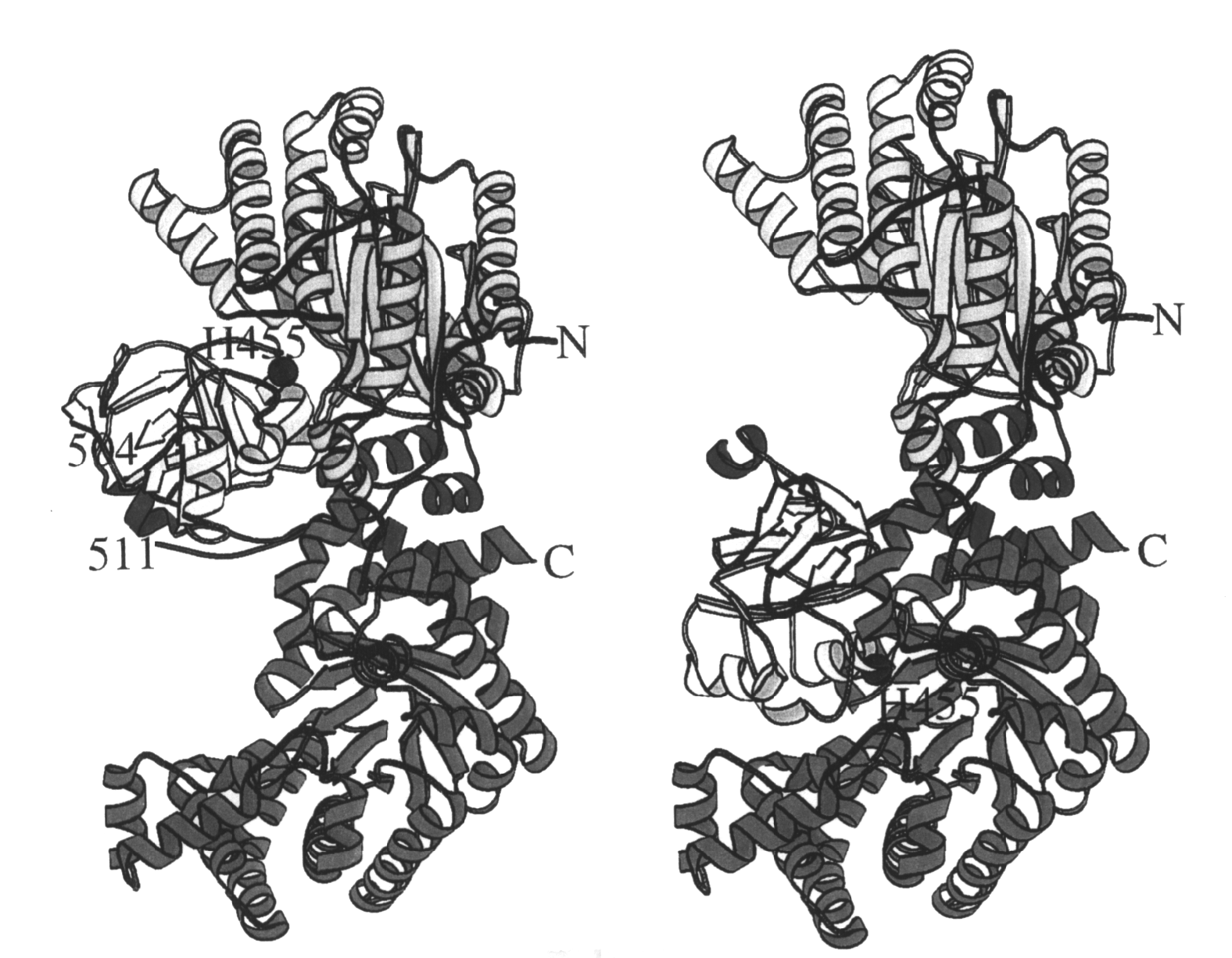
 A third area of study is concerned
with the mechanism and dynamics of domain movement within proteins. For
proteins in which function is coupled to physical translocation the use
of structural domains to mediate movement may be a common solution. For
instance, catalysis at separate active sites in some multienzyme complexes
is linked by a carrier arm, transported by movement of the domain to which
it is appended. In the model system under study in our laboratory, pyruvate
phosphate dikinase (PPDK), catalysis of ATP and pyruvate partial reactions
takes place at two different active sites linked by a carrier histidine.
The active sites are located on the N-terminal and C-terminal domains of
this 3-domain protein. The central domain, containing the carrier histidine,
plays a go-between role between the substrate binding domains. At the present
we are carrying out structural and dynamic studies of the domain movement
occurring during catalytic turnover. The goal of these studies is to learn
how protein-protein and protein-ligand interactions facilitate productive
domain-domain binding.
,
A third area of study is concerned
with the mechanism and dynamics of domain movement within proteins. For
proteins in which function is coupled to physical translocation the use
of structural domains to mediate movement may be a common solution. For
instance, catalysis at separate active sites in some multienzyme complexes
is linked by a carrier arm, transported by movement of the domain to which
it is appended. In the model system under study in our laboratory, pyruvate
phosphate dikinase (PPDK), catalysis of ATP and pyruvate partial reactions
takes place at two different active sites linked by a carrier histidine.
The active sites are located on the N-terminal and C-terminal domains of
this 3-domain protein. The central domain, containing the carrier histidine,
plays a go-between role between the substrate binding domains. At the present
we are carrying out structural and dynamic studies of the domain movement
occurring during catalytic turnover. The goal of these studies is to learn
how protein-protein and protein-ligand interactions facilitate productive
domain-domain binding.
, 
Current Research Funding:
NIH Grant GM 28688 Mechanisms of Enzyme Reactions 2/01/98- 1/31/02
NIH Grant 36260 Investigation of Pyruvate Phoshate Dikinase 4/01/95-3/31/99
 Back to DDM's Group homepage
Back to DDM's Group homepage
 For more information
contact dd39@unm.edu
For more information
contact dd39@unm.edu



