

|
Introduction, Chemistry Review, Cell Biology Review | |
| The scope of anatomy & physiology, the study of the
structures and functions of the human body:
Structure and function are directly related to one another, the concept of which is called their complementarity. The structures: known as the Hierarchy of living organisms. Cells - the simplest structures capable of performing all living functions. Tissues - groups of cells working together to perform specific functions. Organs - tissues working together to perform specific functions. System - a group of organs working together to perform specific functions. This hierarchy illustrates specialization and division of labor which permits the existence of complex organisms such as man. The Functions of Life. 1. Maintaining boundaries and integrity of the organism - illustrated at the cellular level by the cell membrane and at the organism level by the skin. 2. Movement - includes molecular transport, as in the exchange of nutrients and wastes, and movement of the organism or its parts, as in muscular contractions. 3. Responsiveness, irritability, excitability - includes chemical, electrical, and physical responses to stimuli. Illustrated by chemical events at cell membranes and nervous control of muscle contraction and other body functions. 4. Intake of nutrients and digestion - all organisms must take in nutrients and process them. Nutrients include necessary gases such as oxygen or carbon dioxide. 5. Metabolism - the sum of energy producing and energy utilizing chemical reactions. Metabolism converts the energy in nutrients to usable forms and couples that process with energy-requiring processes. 6. Excretion - removal of waste products from the above processes. 7. Growth and repair - organisms must be able to grow to maturity and to maintain and repair damaged tissue in order to survive. 8. Reproduction - organisms must be able to reproduce in order to perpetuate the species. | |
| Homeostasis - defined as a dynamic
balance of processes and materials in the organism.
Homeostasis involves response to stress to maintain a narrow range of conditions, thus sustaining
life.
Most homeostatic mechanisms utilize a control system known as negative feedback. (See Figure 1.4) Negative feedback works like a thermostat. A sensor responds to a variable stimulus. When that variable is outside the normal range the sensor notifies a control center, which then responds directly or triggers a response by an effector. The response has the effect of reducing or negating the original stimulus by bringing the variable back within the normal range. A negative feedback mechanism is self regulating, it turns itself off. Very few processes in the body involve positive feedback and those that do use it to produce a quick response. Some external mechanism is necessary to turn off the process. Examples of positive feedback include: blood clotting, the labor contractions of childbirth, digestion in the stomach. | |
| Chemical constituents of the
human body:
Protoplasm of cells contains water (the major component of all body fluids), electrolytes, O2, CO2, and macromolecules. Extracellular fluid - fluid outside the cells including interstitial fluid (tissue fluid) and blood, together with other specific fluids such as urine and cerebrospinal fluid. Intracellular fluid - the fluid inside cells, also known as the cytosol. Predominant electrolytes in intracellular fluid are K+, Mg+2, Ca+2, H+, PO4-3, SO4-2, HCO3-. Predominant extracellular electrolytes are Na+, Cl-, H+. H+ and HCO3- are important in regulating pH. Hydrogen ions are acid ions and lower the pH of a solution. The pH is important for normal structure and function of proteins. In the human body bicarbonate ions help to neutralize hydrogen ions to maintain a normal pH range. A chemical reaction between carbon dioxide and water in the blood produces carbonic acid in equilibrium with hydrogen ions and bicarbonate. This produces a buffer which maintains the normal pH of the blood. | |
| Chemical
bonds (See Figure 2.8):
Ionic bonds - result from gain or loss of electrons between atoms. This produces ions or electrolytes as above. Ionic bonds are broken when the substance dissolves in water. Covalent bonds - these bonds are produced when electrons are shared between atoms. Polar covalent bonds result when the sharing is unequal. Examples are water and portions of most organic molecules such as carboxylic acids and portions of proteins. Polar molecules have charged portions which attract opposite charges and other polar molecules. Non-polar covalent bonds result when the sharing is equal or the molecule is symmetrical, thus canceling the polarity. Examples include carbon dioxide and long chain hydrocarbons. Hydrogen bonds - are electrical attractions between (NH+) and (C=O-) groups found at distances on the same molecule or on different molecules. Hydrogen bonds cause coiling of proteins and hold the strands of the DNA molecule together, among many examples. | |
| Macromolecules are large complex molecules made from
simpler subunits. There are also
intermediate levels of structure.
Carbohydrates: (See Figure 2.13) simple subunits are monosaccharides or simple sugars such as glucose, fructose, and galactose. Each is a sugar with a molecular formula of C6H12O6. But their structural formulas differ. They are combined by dehydrolysis (dehydration synthesis) to form chains. Dehydrolysis (a.k.a. dehydration synthesis) - the chemical combination of simple molecules into complex forms with the accompanying release of a molecule of water. Two combined in this way (a glycosidic linkage) produce a disaccharide. Two glucose units form maltose, a glucose and a fructose produce sucrose (table sugar), a glucose and galactose form lactose (milk sugar). Continued combining to form a long chain produces a polysaccharide. Polysaccharides include starch and glycogen which are used as stored fuel in plants and animals respectively. The difference between starch and glycogen is the amount of branching with starch being the most highly branched. Cellulose also consists of glucose units but the linkage differs from starch and glycogen and cannot be digested by most animals. For humans cellulose is indigestible fiber, an important component of our diet, but not used for food. Only microorganisms have the necessary enzymes. Termites and other animals that eat wood, grass and the like have the microorganisms in their guts which perform the digestive process known as hydrolysis. Hydrolysis - the splitting of a complex molecule into its subunits by the chemical addition of a molecule of water. Other intermediates: these are intermediates in digestion and are also synthetically produced for use in energy bars and other "athletic foods". dextrins are short portions which include the branch points of polysaccharides. oligosaccharides are 3 to 8 glucose units long. Use of carbohydrates in the human body: Glucose is the primary fuel for most cells and the nearly exclusive fuel for most nervous system cells. Anerobic muscle fibers (those important for strength and speed) depend mostly upon glucose and store glycogen for rapid hydrolysis to glucose. Glycogen is also stored in the liver. The carbohydrate portions of glycoproteins and glycolipids are part of the glycocalyx or "sugar coating" many cells have. This layer is part of the recognition system by which cells are identified by other cells such as those of the immune system, and it functions as a protective coating for other cells. Proteoglycans and glycosaminoglycans (GAGs) are glycoproteins important to the structure and function of connective tissues. [See histology ]. Glycoproteins include collagen, elastin, and reticular fibers. Glycosaminoglycans include hyaluronic acid, heparan sulfate (gives rise to heparin), keratan sulfate (gives rise to keratin), and chondroitin sulfate. | |
| Proteins - made of amino
acid subunits combined by dehydrolysis (See Figure 2.16). The
bond
between amino acids is called a peptide bond and unites them through a nitrogen atom. There are
about 20 naturally occurring amino acids in the body, (See Figure 2.15) eight of which, called essential amino
acids,
must be included in the diet. The others may be manufactured from one another by the liver. Two
amino acids combined produce a dipeptide, more produce a polypeptide. A protein is a
polypeptide
of 100 amino acids or more.
Proteins exhibit 4 levels of structure: (See Figure 2.17) 1o - primary level of structure: the amino acid sequence. This sequence determines the characteristics of the protein and the possibilities are virtually unlimited. All proteins have a primary level of structure. 2o - secondary level of structure: the formation of the alpha-helix. Proteins will coil due to hydrogen bonding between (NH+) and (C=O-) groups on amino acids about four distant. The coils permit protein chains to entwine and braid producing strength especially important in structural proteins. All proteins have a secondary structure, but proteins such as collagen (See Figure 2.18), keratin and elastin as well as Beta pleated sheets, are examples of proteins having a secondary level of structure on which they depend for their functions in the body. 3o - tertiary level of structure: the formation of a specific three-dimensional or globular structure. Tertiary structure results from bending and folding due to hydrogen bonding and the formation of disulfide bonds between cysteine molecules. Because of tertiary structure, proteins can adopt a very precise shape, which is individual to each specific protein. This allows them to act as enzymes, receptors, carriers and the like, molecules which must have a lock-and-key relationship with their substrates or stimulating molecules. (See Figure 2.21). Examples of globular proteins are actin and myosin (proteins important to movement), carrier proteins such as transferrin, which carries iron in the blood, various hormones, enzymes, and other substances such as antibodies. 4o - quaternary level of structure: These proteins have a complex structure and complex functions to match. They are multi-chained (chains must be different) with non-protein cofactors. Hemoglobin is the best known example with several different proteins in its globin part and non-protein heme groups which contain ion atoms for carrying oxygen. Hemoglobin plays a role in both oxygen and carbon dioxide transport and is efficient at loading and unloading these gases. | |
| Lipids: lipids is a category encompassing all
lipid-soluble substances, including fats, steroids,
prostaglandins, eicosanoids, etc.
Fats: Fats are comprised on a glycerol backbone with one, two, or three attached fatty acids (See Figure 2.14). They are stored in the fat reserves of the body for fuel and present as phospholipids in the cell membrane. They are also called mono, di, or triglycerides depending on how many fatty acids are attached (one, two, or three respectively). Fatty acids can vary in length from 4-C to 24-C hydrocarbon chains. If all of these have single bonds (-C-C-) and therefore all possible side bonds have attached hydrogen atoms the fat is said to be saturated. If one or more bonds are double (-C=C-) then the fat is unsaturated. Saturated fat in the diet leads to atherosclerotic plaque in the arteries and diseases such as heart disease and stroke. Saturated fats come mostly from animal fats and are solid at room temperature. Unsaturated fats tend to be metabolized and to not contribute to the artery clogging plaque. Unsaturated fats derive mostly from plant oils and are liquid at room temperature. | |
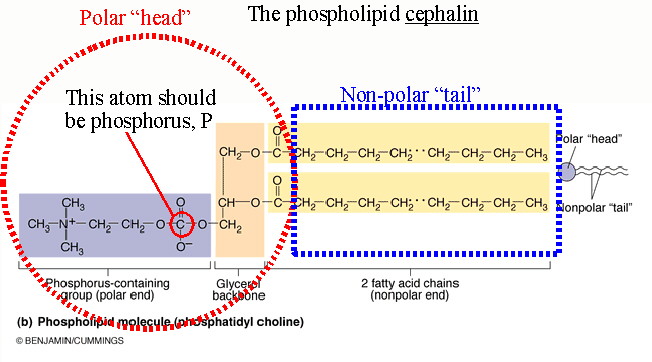 |
A special version of fat is the phospholipid found in cell membranes (See Figure 2.14). Phospholipids and other fats have a polar and a non-polar end. This enables them to act as barriers between aqueous solutions. They line up with their polar "heads" (the glycerol-acid end) toward the water molecules, and their non-polar tails (the fatty acid end) away from the water. For this reason we call the polar "heads" hydrophilic (water loving) and the non-polar "tails" hydrophobic (water fearing). In the cell's membranes they form a phospholipid bilayer which separates the solutions and chemical reactions. |
| The unit
membrane: (See Figure 3.2)
The unit membrane structure, found in the plasma membrane and membrane-bound organelles, is composed of a "fluid mosaic" made up of a phospholipid bilayer with embedded globular proteins. There are integral proteins which act as receptors or carriers or ion channels, and peripheral proteins which might aid in those functions. The fluid mosaic concept reflects the fact that this membrane is changeable and the proteins may move, may increase or may decrease in number and extent. Many of the proteins and lipids are attached externally to carbohydrate and are designated at glycoproteins or glycolipids. The attached carbohydrates form a glycocalyx or "sugar coating" on many cells, especially epithelial cells. Actin fibers on the inside of the cell membrane produce a cytoskeleton which helps to hold the membrane shape and position. The unit membrane: 1) Separates solutions and chemical reactions. It compartmentalizes the cell by forming organelles and the plasma membrane. 2) Allows passive transport by acting as a semipermeable membrane. a) Water soluble substances must pass through the protein channels of the membrane and are restricted according to size or other characteristic. b) Fat soluble substances can pass through the lipid matrix regardless of size. 3) Performs active transport and regulates distribution of molecules and ions across the membrane. 4) Responds to stimuli.
(See Figures 3.6, 3.9, 3.10, 3.11, 3.12, 3.13 for methods of transport) The relationships of the cell's membranes is shown in Figure 3.21, the Endomembrane System. | |
| The Cell:
Organelles (tiny organs) perform the living functions for the cell. The following are membrane-bound organelles, made of the unit membrane structure. Nucleus - (Figure 3.26) contains the DNA. DNA is uncoiled as chromatin (See Figure 3.27) when the cell is not dividing. This is the form in which its message can be transcribed to produce m-RNA. DNA replicates and then coils to produce chromosomes prior to cell division. The m-RNA takes the code to the ribosomes where translation results in protein synthesis. The nucleus is surrounded by the nuclear envelope, a double-layered membrane which connects to the endoplasmic reticulum. Nucleolus (-i pleural) - found in a characteristic number in certain cells. The nucleoli are the source of ribosomal RNA. Ribosomes are the location of protein synthesis. They may be free in the cytoplasm, in which case they make proteins for use inside the cell. Or they may be attached to the endoplasmic reticulum (therefore called the rough endoplasmic reticulum, in which case its proteins will be processed to make substances for secretion by the cell. The endoplasmic reticulum (Figure 3.16) comes in rough and smooth. The rough E.R. makes substances used mostly for cellular secretion. The proteins are made by the attached ribosomes. The smooth endoplasmic reticulum makes and processes non-protein substances, also often for secretion by the cell. Smooth endoplasmic reticulum is also responsible for detoxification of many substances taken in by the cell, and is the location of carbohydrate metabolism including glycolysis. The endoplasmic reticulum is a series of membrane channels within the cell. Golgi bodies ( Golgi apparatus, Figures
3.18, 3.19) assemble the
substances into vesicles (small membrane sacks) for
secretion, or as lysosomes which are used internally. Vesicles excrete
substances by exocytosis, a
process in which vesicles move to the cell membrane and fuse with it, releasing substances to the
outside of the cell. (See Figures 3.10 and 3.11) Substances are
brought into the cell by endocytosis,
a process which forms vesicles in the reverse mechanism of exocytosis. Water and dissolved
substances are brought in by pinocytosis (cell drinking), while particulates
such as bacteria and
cellular debris are brought in the phagocytosis (cell eating). The processes of
endocytosis and
exocytosis are very important to a wide variety of cells. Examples include the lining cells of the
GI
tract, cells of the walls of capillaries, and certain white blood cells and macrophages which
perform
the latter in defending the body against pathogens. Lysosomes contain
digestive enzymes used to
break down phagocytized particulates when the lysosome fuses with the phagocytic vesicle.
Mitochondria - (Figure 3.15) are the site of aerobic metabolism of foods to produce ATP. They contain the enzymes and cytochromes for the Krebs cycle, electron transport system, and other pathways. Mitochondria also contain their own DNA, are the only organelles other than the nucleus to contain DNA, and replicate independently. Aerobic training stimulates skeletal muscle mitochondria to divide to increase the ability of these cells to perform aerobic metabolism. | |
|
Cell Surface Modifications and Non-membraneous
structures:
Cilia - hair-like processes on the apical surface of some epithelial cells which function to move mucus or fluid along the cell surface. The mucus helps to remove particulates and the like, from the respiratory system for example. Cilia are made of microtubules (See Figure 3.22) in a somewhat similar arrangement to those in basal bodies and centrioles. These microtubules link by means of motor proteins to shorten and produce a coordinated wave-like movement. Basal bodies anchor and control movement of cilia. They have microtubules in a specific arrangement (See Figure 3.25) and are derived from centrioles. Flagellum (-a) - the tail-like process which aids in sperm motility. Centrioles - (Figure 3.24) are structures within the cell which attach to spindle fibers to move the chromosomes during cell division. Centrioles are also made of the same microtubule array as seen in basal bodies and similar to the cilia and flagella. Microvilli - These are cytoplasmic processes that extend from the cell's surface. They vary from irregular and small in some cell types, to tall, closely packed, uniform projections that provide enormous surface area of other cells. Microvilli are more abundant and uniform in cells whose primary function is secretion and absorption. Stereocilia are simply unusually long microvilli. Microvilli are typically supported internally by actin filaments. The cytoskeleton and other supporting components: microfilaments: composed of thin actin filaments. These are found beneath the plasma membrane where they aid in cell shape and movement (e.g. in macrophages which exhibit amoeboid movement). [See also the zona adherens below.] Intermediate filaments - these are keratin filaments which primarily form the desmosomes and hemidesmosomes. Microtubules - made of tubulin protein, a globulin protein, arranged into coils which produce the tubules. Microtubules are important for support and for transport within the cell. | |
| Cell junctions: (See Figure
3.4: [4th Edition|5th
Edition]
Epithelial cells, certain muscle cells, and others which lie side by side have specialized connections between them, called a junctional complex. In epithelium this connection is particularly significant because it allows a sheet of cells to create a barrier, thus compartmentalizing a tissue and restricting passage of substances across the epithelium. Tight junctions (a.k.a. zona occludens) are most important in restricting transport across an epithelial membrane, for instance in the wall of a capillary. Tight junctions are fusions of adjacent plasma membranes and vary somewhat in how "tight" they are. Depending on the extent and complexity of the fusions, some allow water and small molecules to pass through gaps between them (e.g. in the kidneys), and others allow very little to pass. In this way tight junctions allow these epithelial sheets to act as semipermeable membranes. Desmosomes are anchoring junctions with keratin filaments (a.k.a. intermediate filaments) running between the cells. These filaments often run across the cell laterally and are important in tissues where the cells are subjected to lateral stress, such as the skin or lining of the urinary bladder. Desmosomes are also known as macula adherens and are like spot welds between adjacent cells (macula is Latin for 'spot'). There are also zona adherens which are more continuous adhesions composed of actin microfilaments that anchor cells to one another at the apical end. Hemidesmosomes (half-desmosomes) are small bundles of keratin filaments which anchor the basal membrane of an epithelial cell to the underlying connective tissue. Gap junctions have channels called "connexions" which allow ions and, therefore, electrical impulses to pass from one cell to the next. Gap junctions are found in many tissues, but the best examples is between cardiac muscle cells in the heart and smooth muscle cells in the intestine. | |
NEXT: Histology