Research Interest
Synthetic Organic Chemistry and Chemical Biology
Our research lies at the interface of chemistry and biology. Chemical synthesis plays an increasingly significant role in the advancement of life sciences. Our research program aims to advance this important paradigm. Inspired by Nature’s success in generating functional molecules in an efficient and environmentally friendly way, we are undertaking a biological approach to synthesis in which the synthetic strategies and process are combined with the principles and tools of chemistry to create molecules with new functions. The main emphasis is on discovery of new catalytic reactions, and efficient assemble of diverse and medicinally important chemical structures. The parallel objective is to bring together organic synthesis, molecular and cell biology and biochemistry in order to enable the development of an arsenal of functional small-molecules for basic and translational biomedical research. By studying the properties of the resulting molecules, new insights are gained into the molecular mechanisms of complex biological and chemical systems. Furthermore, we also perform medicinal chemistry to improve the potency, cellular activity, specificity, stability and pharmacological properties of our initial "lead" compounds.
1. Synthetic Organic Chemistry: Development of organocatalytic, enantioselective organic reactions.
Although organic synthesis has reached a stage, where any desired molecule can be synthesized given ample time, resource and skilled personnel, challenges related to improving synthetic efficiencies remain important. Accordingly, the development of new, efficient synthetic strategies for the construction of complex molecules is of considerable significance in the context of contemporary organic synthesis. In biocatalysis, enzymes catalyze reactions, most often involving several sequential steps in one-pot transformation (e.g., tandem process), that enable to produce complex structures with high efficiencies and specificities. In the past few years, small organic molecule-based catalysis (termed “organocatalysis”) has emerged as a new frontier in catalysis and interest in organocatalysis has increased spectacularly in the last few years as a result of both the novelty of the concept and, more significantly, because of the fact that the operational simplicity, less toxicity, efficiency and selectivity make many organocatalytic reactions superior to those carried using conventional methods. Therefore, the development of organocatalytic enantioselective tandem processes is even more appealing. It is especially significant when the organocatalyzed tandem processes produce synthetically and medicinally important “privileged” structures, which have been identified as core structures in numerous therapeutics and natural product targets. The goal of the research program is to develop organocatalyzed enantioselective tandem reactions that generate diverse, structurally and stereochemically complex “privileged” molecule skeletons in highly concise fashions.
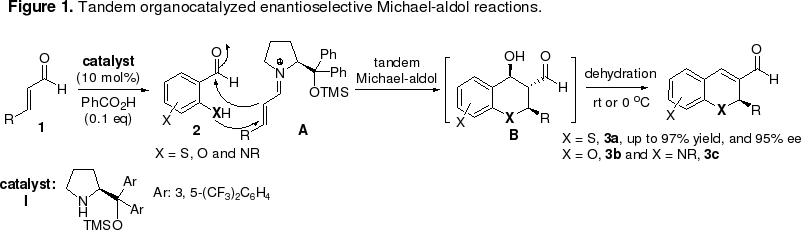
Organocatalytic reactions are ideally suited for the design of tandem processes since a single organocatalyst can often promote several types of chemical transformations. For example, an amine can participate in formation of a nucleophilic enamine from an enolizable aldehyde/ketone and an electrophilic iminium ion from a,b-unsaturated aldehyde/ketone, and in this way generate two complementarily reactive species. By rational design, it should be possible to integrate the two catalysis principles in tandem reaction sequences. Along this line, we have planed to develop organocatalytic asymmetric Michael-aldol and -Mannich and Michael-Michael reactions. These one-pot methods will enable facile access to the “privileged” structures such as thiobenzopyrans (3a), benzopyrans (3b), and hydroquinolines (3c) (Figure1). Recent effort from our laboratory has resulted in the discovery of a new organocatalyzed enantioselective tandem thio-Michael-aldol reaction that furnishes benzothiopyrans 3a in high yields (81-97%) and with good to high enantioselectivities (85-94% ee) (for detail see: J. Am. Chem. Soc., 2006, 128, 10354-10355) (Figure 1).

A significant merit of Michael addition reactions is that a wide variety of substances can serve as acceptors (electrophiles) and donors (nucleophiles) and, consequently, a diverse array of products can be generated (for an example, please see our recent publication: J. Am. Chem. Soc., 2006, 128, in press). A host of substrates can be explored for their participation in tandem Michael-aldol, Michael-Mannich, and Michael-Michael processes. In addition, we are also interested in the development of organocatalytic, enatioselective tandem Friedel-Crafts-aldol, and aldol-lactionization, and lactamization, and multicomponent tandem reactions. The syntheic value of these tandem reactions will be demonstrated in natural product synthesis and diversity oriented library synthesis (see below).
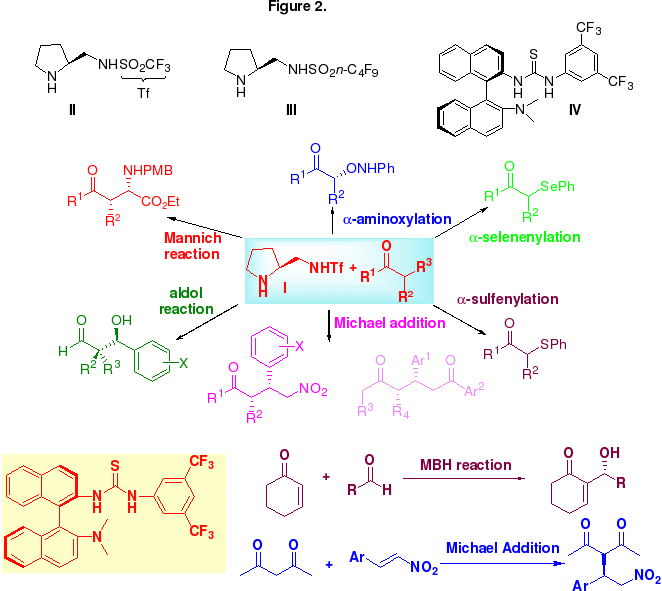
With the scope of organocatalysis rapidly expanding, it is important to fully recognize the limitations and disadvantages of the catalysts that have been uncovered thus far. First, as compared with organometallic catalysts, only a small number of organic reactions have been promoted by organocatalysts. Second, even in successful cases high catalyst loadings (10-20 mol%) of organocatalysts are generally required to effect the desired reactions in reasonable timescales. Third, although organocatalysis can be considered as a mimic of enzyme-promoted reactions, to date the majority of organocatalyzed reactions are performed in organic solvents rather than in water. In this regard, the second goal of the research effort is to develop new recyclable and reusable forms of organocatalyst and ones that will promote reactions in water. Recently, we have developed two new classes of organocatalysts: chiral pyrrolidine sulfonamides II-III and binaphthyl-based amine thiourea IV for catalyzing a wide range of enantioselective organic transformations (Figure 2). More recently, we have found the perfluorous chiral pyrrolidine sulfonamide III, which can be recovered by simple fluorous solid-phase extraction and reused up to 6 cycles without a significant loss activity and stereoselectivity toward Michael addition reaction of aldehydes and ketones to nitroolefins. More importantly, the catalytic process can proceed in pure water. Our continuing effort in this field is to utilize these catalysts and design their fluorous version and new organocatalysts for new organic reactions.
The significant work has attracted considerable interest from academic and industrial laboratories. For example, two recent Org. Lett. papers (Org. Lett., 2005, 7, 4293-4296 and Org. Lett., 2005, 7, 4713-4716) have been recognized as Org. Lett. Hot Paper (only total 7 papers in this category), and one Org. Lett. paper (Org. Lett., 2005, 7, 601-604) has been recognized as one of the most accessed articles of Org. Lett. in year of 2005.
2. Functional small molecule discovery: Probes for understanding biological functions of macromolecules in biological systems and therapeutic agents in drug discovery.
The utilization of small organic molecules has been proved to be a particularly powerful tool for study of biological systems. In contrast to genetic knockouts and RNA knockdowns, selective small molecule probes can be used to study the individual functions of multifunctional proteins and can distinguish between different conformational and post-translational modification states of their targets. We are developing an innovative and general strategy by the selection of "privileged" structures as lead compounds in conjunction with diversity oriented library synthesis to build complex molecules. The structures and functions of natural products suggest that structural complexity may be positively correlated with macromolecule-perturbing function and specificity of action. This correlation is particularly striking in small molecules known to disrupt protein-protein interactions. Therefore, the strategy we employ can allow to rapidly identify potent ligands to receptors and inhibitors to enzymes.
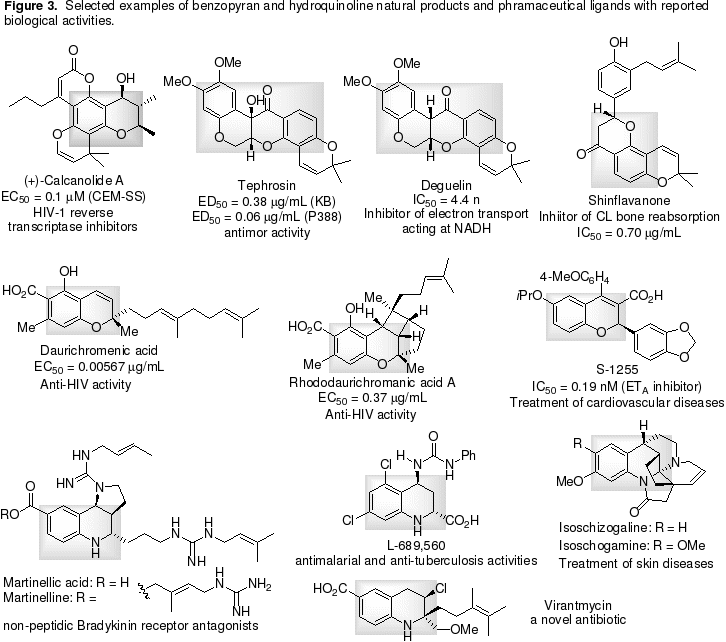
Chiral thiobenzopyrans, benzopyrans and hydroquinolines (Figure 3) are medicinally and biologically important “privileged” scaffolds, which have been found in numerous biologically active molecules. Some examples of natural products and synthetic substances are described in Figure 3.
In synthetic effort, we employ these new efficient, organocatalytic, enantioselective reactions described above for synthesis of: 1) complex natural products and drugs, 2) the “privileged” structures, which can be further modified by diversity oriented library synthesis for biological studies, and 3) the biologically active analogues.
2.1. Efficient total syntheses of complex natural products and therapeutic agents. Recently, we have demonstrated that these organocatalytic, enantioselective reactions we have developed have served as key steps in our total synthesis of natural product trichostatin A, a potent and selective inhibitor of histone deacetylases (HDACs) (Adv. Synth. Catal., 2006, 348, 1228-1234) and drug candidate Sch-50971 (Chem. Eur. J., 2006, 12, 4321-4332), a potent H3 agonist with potential use for the treatment of obesity and Alzheimer’s disease. Current and continuing efforts focus on the synthesis of the analogues of trichostatin A, and natural products martinellic acid and martinelline, a non-peptidic bradykinin receptor antagonist, (+) calcanolide A (in clinical trial) and (+) inophyllum, potent HIV RP inhibitors and synthetic substance S-1255, a potent and selective ETA inhibitor, which currently in clinical trials (their structures shown in Figure 3).
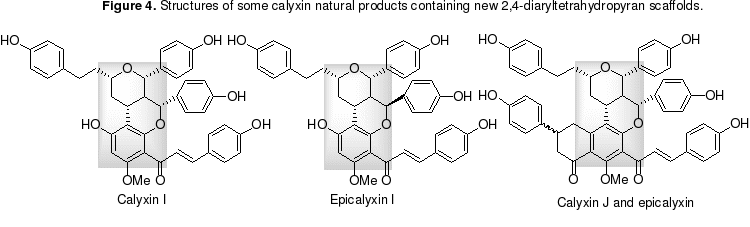
A new class of natural products was isolated recently from the Alpinia blepharocalyx seeds, which have been used for the treatment of stomach disorders in Chinese medicine. Several of these natural products display biologically interesting antiproliferative activity against carcinoma cell lines (Figure 4). Calyxin I, the most potent member of the class, showed 0.89 mM inhibition activity toward HT-1080 fibrosarcoma and colon 26-L5 carcinoma. Members of this natural product family, which share a unique, fused two-tetrahydropyran scaffold, have begun to receive interest from the synthetic community. We plan to employ organocatalytic, enantioselective oxa-Michael-aldol reactions as a critical step to efficiently construct the chiral benzopyran scaffold in calyxin I, and its analogues for structure-activity relationship study and its other family members.
2.2. Efficient creation of functional “privileged” structure-based small molecule libraries using diversity oriented synthesis and structure-based rational design of ligands, and the investigation of their functions in biological systems.
Small molecules have been shown as extremely powerful tools for studying biological systems. A significant challenge to be addressed in chemical biology and drug discovery is the rapid identification of new, highly specific small molecule “leads”. We are engaged in a two-pronged approach to this problem involving both diversity-oriented synthesis and computer-aided rational ligand design. In the first approach, novel combinatorial libraries are synthesized and used in high-throughput screens against a variety of biological targets. In the second approach, structural and mechanistic information about a selected biological target is used to guide the design of individual small molecule probes. These two approaches are complementary and, indeed, can often be combined productively.
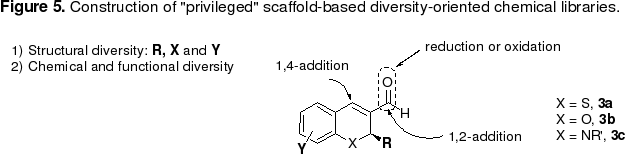
Our efforts in diversity-oriented synthesis are focused on generating libraries based on privileged structural motifs found in biologically active natural products. Such structures have a demonstrated ability to bind multiple classes of biological targets. Further, in contrast to widely available 'drug-like' libraries, these natural product-like libraries exhibit much greater structural diversity and complexity. As mentioned above, chiral benzothiopyrans, benzopyrans and hydroquinolines are a class of “privileged” scaffolds (Figure 5). Our organocatalytic, enantioselective tandem Michael-aldol reactions can produce these scaffolds in one-pot transformation from readily available starting materials. These functionally and structurally diversified scaffolds (1a-c) are ideal structures for further elaboration in the diversity-oriented synthesis (Figure 5). We leverage multidisciplinary collaborations with biologists to carry out biological evaluation of the molecules we synthesize, particularly in the areas of cancer neurodegenerative and infectious diseases.
In the rational design of ligands, currently we focus on the development of PDE2, XIAP, BRCA1 and HIV integrase inhibitors. These projects are carried out with collaboration with computational chemists and biologists. We have found potent and selective ligand targeting these macromolecules. Further effort directs toward to understanding structure-activity relationship and improving the potency, cellular activity, specificity, stability and pharmacological properties to obtain clinically useful “drug-like” molecules.