
 |
|||||||||||||||
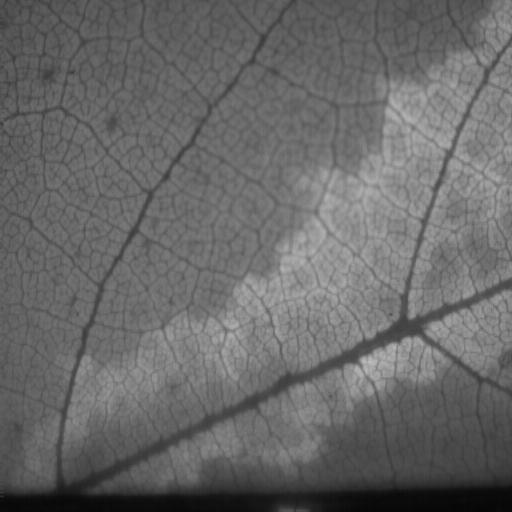 Dogma
suggests that under most circumstances, photosynthetic rates are fairly uniform
across leaves. However, tools to measure this variation have only recently become
accessible for a wide range of studies. Patchiness of photosynthetic function
is beginning to get more attention and though early work has documented examples
of it, the conditions that cause patchiness are not yet clear. My lab, in a
collaboration with Dr. Pockman's lab here at UNM, is using chlorophyll fluorescence
imaging (the primary tool for assessing patchiness) to examine photosynthetic
patchiness under a range of conditions that alter plant hydraulic conductance.
This collaboration was initiated as a class project on plant stress and is now
a major project for one of our Ph.D. students.
Dogma
suggests that under most circumstances, photosynthetic rates are fairly uniform
across leaves. However, tools to measure this variation have only recently become
accessible for a wide range of studies. Patchiness of photosynthetic function
is beginning to get more attention and though early work has documented examples
of it, the conditions that cause patchiness are not yet clear. My lab, in a
collaboration with Dr. Pockman's lab here at UNM, is using chlorophyll fluorescence
imaging (the primary tool for assessing patchiness) to examine photosynthetic
patchiness under a range of conditions that alter plant hydraulic conductance.
This collaboration was initiated as a class project on plant stress and is now
a major project for one of our Ph.D. students.
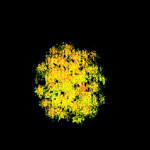 Bryophyte
photosynthesis is generally measured using a petri-dish size sample in a gas
exchange system. This has two major problems. First, the photosynthetic rate
measured is the average rate of a population of plants that are almost always
experiencing different micro-environments (especially regarding wind and light).
Second, most systems require detaching samples from their substrate which significantly
alters their structure, including intricate connections between the moss and
its substrate (see my bryophyte research page for
more info). Photosynthetic rates of bryophytes are highly dependent on water
content and the disruptions caused by pulling up samples has an enormous affect
on the way the samples dry. Chlorophyll fluorescence imaging solves both of
these problems. Images of photosynthesis can provide small scale
Bryophyte
photosynthesis is generally measured using a petri-dish size sample in a gas
exchange system. This has two major problems. First, the photosynthetic rate
measured is the average rate of a population of plants that are almost always
experiencing different micro-environments (especially regarding wind and light).
Second, most systems require detaching samples from their substrate which significantly
alters their structure, including intricate connections between the moss and
its substrate (see my bryophyte research page for
more info). Photosynthetic rates of bryophytes are highly dependent on water
content and the disruptions caused by pulling up samples has an enormous affect
on the way the samples dry. Chlorophyll fluorescence imaging solves both of
these problems. Images of photosynthesis can provide small scale  detail
only limited by the resolution of your camera and the size of the area to be
imaged. This means that variation between plants and even between branches and
leaves on a single moss can be distinguished (see my fluorescence
page). In addition, these data can be collected without even touching the
bryophytes, thereby minimizing artifacts from disrupting community structure.
I have been collaborating with Dr. Steven Rice from Union College on a project
where we are assessing the affects of Sphagnum architecture on photosynthetic
function, light attenuation, and boundary layer conductance by combining his
3-D imaging techniques with gas exchange and fluoresence imaging.
detail
only limited by the resolution of your camera and the size of the area to be
imaged. This means that variation between plants and even between branches and
leaves on a single moss can be distinguished (see my fluorescence
page). In addition, these data can be collected without even touching the
bryophytes, thereby minimizing artifacts from disrupting community structure.
I have been collaborating with Dr. Steven Rice from Union College on a project
where we are assessing the affects of Sphagnum architecture on photosynthetic
function, light attenuation, and boundary layer conductance by combining his
3-D imaging techniques with gas exchange and fluoresence imaging.
Cyanobacterial dominated desert soil crusts are some of the most difficult samples to analyze for photosynthetic function. First, they are ephemeral in nature - potentially only active for hours at a time. Second, they are extremely fragile, so they cannot be touched without damaging them. Third, they have low photosynthetic rates per unit area, and finally they are difficult to maintain in culture. Fluorescence imaging has great promise for measuring photosynthetic function in the field since the measurements are quick, the crust doesn't need to be touched, and it gives information spatial variation. However, standard fluorescence tools for plants have to be altered for good cyanobacterial measurements due to interference from the phycobilisome pigments. Also, cyanobacterial photosynthetic electron transport and respiratory electron transport are integrated which greatly complicates and limits interpretation of the data. Still, much can be learned from this technique and no other methods are better. We currently have an NSF funded project (a collaboration with Dr. Robert Sinsabaugh of UNM, Dr. Scott Collins of UNM, and Dr. Michael Allen of UC-Riverside) to examine nitrogen cycling in desert grasslands at the Sevilleta Long Term Ecological Research Station and we are using chlorophyll fluorescence imaging to assess photosynthetic activity of the crusts.