
 |
|||||||||||||||
Stable isotopes are extremely useful for studying a wide range of biological processes either through monitoring changes in natural abundance or through enriching samples with one isotopomer and tracking how the label moves through a system. I utilize both approaches in my research, sometimes via traditional isotope ratio mass spectrometry, but more often I use two powerful isotopic tools that are only available at a few institutions world-wide: 1) tunable diode laser absorbance spectrometry and 2) membrane inlet mass spectrometry.
 TDL
technology has been in use for over a decade, but it is only within the last
couple years that it has been fine tuned for use in measuring the natural abundance
of CO2 isotopomers in the atmosphere. This tool is unlike an isotope ratio mass
spectrometer because it measures the absolute concentration of 13CO2 and 12CO2
in atmospheric samples instead of the ratio of the isotopomers. It does this
by detecting absorbance in very narrow portions of the infra-red spectrum that
are specific to each isotopomer and the precision is near that routinely achieved
with mass spectrometry. A major advantage of the TDL is that high precision
isotopic concentrations can be determined every 15 seconds in a flowing stream
of air rather than by collecting gas in a flask for 30 minutes to 2 hours and
then measuring the isotope ratio on a mass spectrometer. This high frequency
not only allows the study of dynamic processes with two orders of magnitude
greater time resolution than has ever been achieved, but it also greatly simplifies
the measurement process and the real-time output gives the researcher the capability
of troubleshooting during data collection.
TDL
technology has been in use for over a decade, but it is only within the last
couple years that it has been fine tuned for use in measuring the natural abundance
of CO2 isotopomers in the atmosphere. This tool is unlike an isotope ratio mass
spectrometer because it measures the absolute concentration of 13CO2 and 12CO2
in atmospheric samples instead of the ratio of the isotopomers. It does this
by detecting absorbance in very narrow portions of the infra-red spectrum that
are specific to each isotopomer and the precision is near that routinely achieved
with mass spectrometry. A major advantage of the TDL is that high precision
isotopic concentrations can be determined every 15 seconds in a flowing stream
of air rather than by collecting gas in a flask for 30 minutes to 2 hours and
then measuring the isotope ratio on a mass spectrometer. This high frequency
not only allows the study of dynamic processes with two orders of magnitude
greater time resolution than has ever been achieved, but it also greatly simplifies
the measurement process and the real-time output gives the researcher the capability
of troubleshooting during data collection.
 Dr.
Nate McDowell in the Earth and Environmental Sciences division of Los Alamos
National Laboratories has recently acquired a TDL and we have been collaborating
to make the world's first system that couples isotopic analyses with standard
gas exchange measurements of photosynthesis by plants and algae in real-time.
Adding isotopic analyses to standard gas exchange measurements is a robust way
for measuring the diffusion of CO2 within leaves (a poorly understood process)
and for assessing the activity of CO2 concentrating mechanisms (see my ccm
page). Our collaboration will link apparent photosynthetic and respiratory
fractionation to atmospheric isotopomer composition, thereby improving the inferences
we can make about ecosystem function using isotopic analyses. As part of this
research we are collaborating with Dr. Margaret Barbour of Landcare Research
in New Zealand to link isotopic composition of respired CO2 with that of sucrose
transported in the phloem which is providing new insight into interactions between
photosynthesis and respiration. Finally, Dr. McDowell has recently upgraded
his TDL to allow real-time analyses of the 18O and 16O content of CO2. This
taps into a whole new developing field studying the processes that control 18O
content of CO2 and water in plants and ecosystems and this will be a major area
of study for our group.
Dr.
Nate McDowell in the Earth and Environmental Sciences division of Los Alamos
National Laboratories has recently acquired a TDL and we have been collaborating
to make the world's first system that couples isotopic analyses with standard
gas exchange measurements of photosynthesis by plants and algae in real-time.
Adding isotopic analyses to standard gas exchange measurements is a robust way
for measuring the diffusion of CO2 within leaves (a poorly understood process)
and for assessing the activity of CO2 concentrating mechanisms (see my ccm
page). Our collaboration will link apparent photosynthetic and respiratory
fractionation to atmospheric isotopomer composition, thereby improving the inferences
we can make about ecosystem function using isotopic analyses. As part of this
research we are collaborating with Dr. Margaret Barbour of Landcare Research
in New Zealand to link isotopic composition of respired CO2 with that of sucrose
transported in the phloem which is providing new insight into interactions between
photosynthesis and respiration. Finally, Dr. McDowell has recently upgraded
his TDL to allow real-time analyses of the 18O and 16O content of CO2. This
taps into a whole new developing field studying the processes that control 18O
content of CO2 and water in plants and ecosystems and this will be a major area
of study for our group.
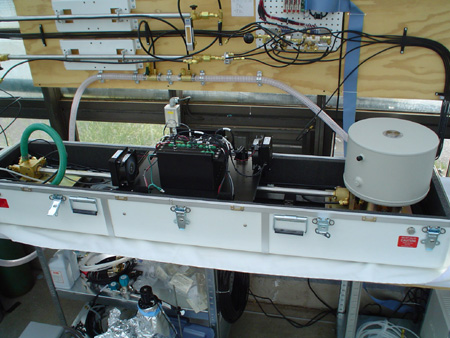
 Membrane
inlet mass spectrometry (MIMS) is a powerful technique that allows simultaneous
monitoring of a wide range of isotopomers in a gas sample (generally a closed
system, but some applications work with open system or near open system gas
exchange). In essence, a plastic membrane is used to separate the contents of
a cuvette (leaf tissue plus air, or a liquid algal culture) from the detector
of a mass spectrometer. The plastic allows a high vacuum to form on the mass
spectrometer side which then slowly pulls gases through the plastic membrane
and into the mass spectrometer for analysis. This tool is only used by a handful
of labs world-wide and we are in the process of setting up a system here at
UNM through a collaboration with Dr. Zachary Sharp in the Earth and Planetary
Sciences department and Dr. Anthony Martino at Sandia National Laboratories.
Membrane
inlet mass spectrometry (MIMS) is a powerful technique that allows simultaneous
monitoring of a wide range of isotopomers in a gas sample (generally a closed
system, but some applications work with open system or near open system gas
exchange). In essence, a plastic membrane is used to separate the contents of
a cuvette (leaf tissue plus air, or a liquid algal culture) from the detector
of a mass spectrometer. The plastic allows a high vacuum to form on the mass
spectrometer side which then slowly pulls gases through the plastic membrane
and into the mass spectrometer for analysis. This tool is only used by a handful
of labs world-wide and we are in the process of setting up a system here at
UNM through a collaboration with Dr. Zachary Sharp in the Earth and Planetary
Sciences department and Dr. Anthony Martino at Sandia National Laboratories.
Direct in vivo comparison of PSII activity and Rubisco activity
A major advantage of membrane-inlet mass spectrometry is the ability to measure photosynthetic gas exchange in both liquid and gas samples, which means any photosynthetic organism (from cyanobacteria to crop plants) or any tissue extract can be studied with this single instrument. Membrane-inlet analyses provide the only way to directly measure photosystem II (PSII) activity in vivo. This is accomplished by monitoring 16O2 (mass 32) generated by the oxygen evolving complex from leaf water (H-16O-H) after enriching the leaf chamber O2 with doubly labeled 18O2 (mass 36). These data can also be used to simultaneously estimate photorespiration and CO2 (mass 44) exchange rates in order to compare rates of Rubisco activity with electron transport (Hanson et al., 2003).
Carbonic anhydrase activity assay
Another extremely important use of membrane-inlet mass spectrometry is in a sensitive assay for carbonic anhydrase activity. This assay uses 13C and 18O doubly labeled bicarbonate and monitors changes in the rate of depletion of mass 49 (13C18O2) to 47 (13C18O16O) and 45 (13C16O2). The presence and specific activity of carbonic anhydrase in crude tissue extracts is determined by increases in the rate of depletion. The major advantage of this method is that, unlike the standard Wilber-Anderson technique, this assay can be done at any temperature. The ability to test for activity at biologically relevant temperatures has already led to the discovery of carbonic anhydrase activity in samples where activity was previously believed to be missing.