|
| |
Vitamin C: Which contains more, citrus fruits or
green vegetables? Does it decompose over time?
Project Purpose
The purpose of this experiment is twofold. First, to detect and
compare the concentration of vitamin C in several citrus fruit and green vegetable juices
by titration of the juice with a corn starch-iodine vitamin C indicator solution;
and second, to study the effects of (1) heat, (2) freshness, and (3) sunlight on the
concentration of vitamin C in them.
School Name: Monzano Day School
Grade: Fourth
Teacher: Heather Behling
How did I get interested in the project?
- A few years ago, I thought the "C" in vitamin C stood for the
word "citrus" because television commercials related vitamin C to citrus fruit
juices, for example, orange juice. In recent years, I started thinking that the
"C" in vitamin C actually stood for the word "cold" because my mom
generally asks me to take a vitamin C tablet everyday when I catch a cold. She knows very
well that fruit juices do not exist in my list of favorites. Vitamin C tablets are not my
favorites either, so I would like to scientifically investigate what else I can eat/drink
to substitute these tablets or citrus fruit juices.
- In 1994, Dr. Linus Pauling, the late American scientist, two time Nobel
prize winner, and humanitarian, claimed, "vitamin C has the potential to prevent and
treat common cold, flus, and cancer, all of which plague our society." This created a
lot of interest in me to learn more about the topic.
- I like science, especially chemistry is fascinating to me because in many
experiments one can see changes in colors taking place due to chemical reactions.
- A science experiment book suggested that a corn starch-iodine solution,
which is royal blue in color when properly prepared, could be used to detect vitamin C in
food juices, and I thought I would investigate it myself.
- This is a fun project for me and my entire family.
The Scientific Hypotheses I would test:
Hypothesis I:Some green vegetables contain more vitamin C than citrus fruits
(which is contrary to the popular belief).
Hypothesis II: Vitamin C decomposes over time, on exposure to sunlight, and
heat.
The Scientific Background
For our body and mind to stay healthy, we need to eat a balanced
diet, which should include the four food groups, vitamins and minerals. The four food
groups include the milk group, fruits and vegetables, meat group, and breads and cereals.
The vitamins include A, B1, B2, B12, C, D, E, and K. The
minerals include calcium, iodine, iron, phosphorous, potassium, and sodium.
One of the most important vitamins in our diet is vitamin C (which is also known as
ascorbic acid), found in many fruits and vegetables. Vitamin C is also easily damaged or
destroyed.
The best-known sources of vitamin C are the citrus fruits – oranges,
lemons, limes, tangerines, and grapefruits. Good vegetable sources include peppers,
tomatoes, parsley, dark leafy greens, and cabbage. Animal foods contain almost no vitamin
C.
Vitamin C has many functions. It is needed for healthy bones, teeth, and gums;
growth; strength of blood vessels; fast healing of wound; and increasing the body’s
resistance to infection.
In this experiment, I will scientifically test the concentration of vitamin C in a
vitamin C tablet (the control sample) and in several citrus fruits and green vegetables.
To determine the concentration of a substance in a solution, chemists use the technique
called titration. This means adding, in precise measured amounts, a reacting agent
of known concentration until the solution changes color, indicating a chemical reaction. I
will prepare a water solution of a vitamin C tablet, and juices of fruits and green
vegetables. The reacting agent in my experiment will be the corn starch-iodine vitamin C
indicator solution.
There are three controlling variables in this experiment. First, the correct
preparation of corn starch-iodine vitamin C indicator solution, and since this solution is
unstable, it is freshly prepared each time it is used. Second, the amount of corn
starch-iodine vitamin C indicator solution used for each test sample did not vary during
the experiment. And finally, I made a control sample of Vitamin C tablet solution to
determine whether a weaker or stronger concentration of vitamin C was present in each
fruit and vegetable juice tested.
Glossary of Terms Used
| Chemistry |
the study of matter. |
| Chemist |
one who studies the make ups and properties of
substances and investigates how substances react with one another. |
| Chemical reaction |
a change that produces one or more new
substances. |
| Comparing |
observing how things are alike or different. |
| Controlling variables |
identifying and managing factors that may
influence the accuracy of an experiment. |
| Experiment |
testing under controlled conditions. |
| Hypothesis |
tentatively accepting an explanation as the
basis for further investigation. |
| Inferring |
implying a conclusion from available evidence. |
| Interpreting data |
finding patterns or relationships in a set of
data. |
| Titration |
adding, in precise measured amounts, a
reacting agent of known concentration until the solution changes color (in our experiment,
from royal blue to clear), indicating a chemical reaction. |
| Vitamins |
organic substances for the regulation of the
metabolism and normal growth and functioning of the body. |
What materials did I use?
- 40 clear plastic cups
- 40 plastic stirrers
- 1 medicine dropper
- food processor
- large wire strainer
- hot plate
- cooking pot
- one 250 ml measuring cup
- two small bowls
- one gallon of distilled water
- starch-iodine vitamin C indicator
- two teaspoons of corn starch
- tincture of iodine
- Juices (some prepared and some ready made) of several citrus fruits and
green vegetables
- one 100 mg vitamin C tablet
What did I do?
- Prepared the juices that were not ready made and stored them in the
refrigerator in cups.
- Prepared a vitamin C standard. Placed a 100 mg vitamin C tablet in a
small plastic bag and crushed it with a hammer into a powder. Added the powdered vitamin C
into 100 ml of distilled water and mixed them well. This solution was then poured into a
closed container and stored in the refrigerator.
- Prepared the corn starch-iodine vitamin C indicator solution. Poured half
a teaspoon of corn starch into the cooking pot. Poured a cup of distilled water into the
pot. Heated and stirred the pot of water and corn starch solution until all the cornstarch
is dissolved in the water. After heating, I let everything cool. Poured two teaspoons of
the solution and one cup of distilled water into one of the cups. Using the medicine
dropper, added four drops of tincture of iodine into the solution. The color of the
resulting solution was royal blue. At that point, the corn starch-iodine test solution was
done.
- Titration of the vitamin C standard and the fruit and vegetable juices
one at a time. The procedure for the titration was carried out using the following steps.
Step 1: placed 25 ml of the corn starch-iodine indicator solution into a clear plastic
cup. Step 2: used the medicine dropper to drop in the vitamin C standard or the juice of
fruit or vegetable being tested. Step 3: after each drop, I stirred the resulting
solution. Step 4: when the royal blue indicator changed to clear, I stopped adding the
juice. Step 5: recorded the number of drops needed to change the royal blue indicator to
clear. Step 6: repeated steps 1-5 above for each item being tested.
- Poured a small amount of the vitamin C standard or the juice of each item
being tested into two clear plastic cups. Placed one of the cups on a table under shade
for one hour. Placed the other cup on a table under direct sunlight for one hour.
Titration of the content of each cup was performed and the result recorded.
- Heated one cup of each item being tested, one at a time, to boiling and
let it boil for about 10 minutes. Let the item to cool down, and performed the titration
procedure and recorded the results.
What happened?
The results obtained from the experiment performed above are
recorded in the table below. These results are captured and analyzed through the four bar
charts presented below.
|
Conditions of Test Samples |
|
|
Freshly Prepared/ Opened Can of Juice |
Fresh Juice Exposed to the Air for 1 hour Under Shade |
Fresh Juice Exposed to the Air for 1 Hour Under Direct Sunlight |
Item Tested |
Number of Drops Needed for Titration |
Number of Drops Needed for Titration |
Number of Drops Needed for Titration |
Vitamin
C Standard |
6 |
7 |
8 |
Orange |
8 |
9 |
9 |
Lemon |
8 |
14 |
17 |
Grapefruit |
12 |
15 |
15 |
Lime |
14 |
16 |
18 |
Tomato |
13 |
15 |
18 |
Green
Pepper |
4 |
6 |
6 |
Parsley |
5 |
6 |
7 |
Collard
Green |
5 |
6 |
8 |
Mustard
Green |
7 |
10 |
15 |
Cabbage |
9 |
11 |
13 |
NM
Green Chile |
3 |
8 |
11 |
NM
Red Chile |
1 |
3 |
4 |
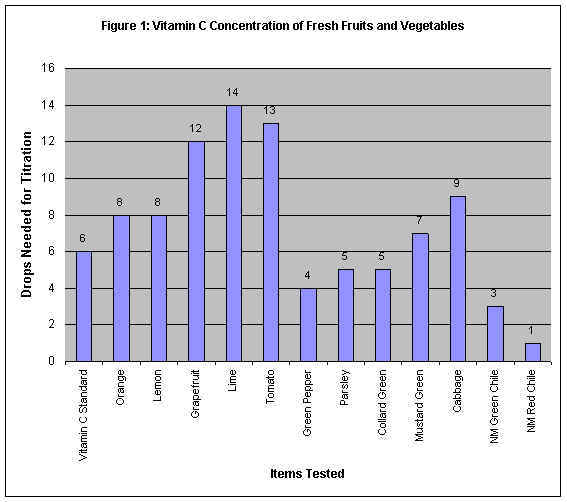
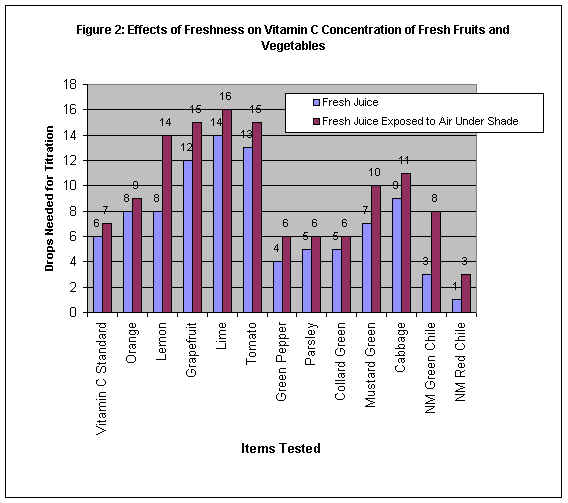
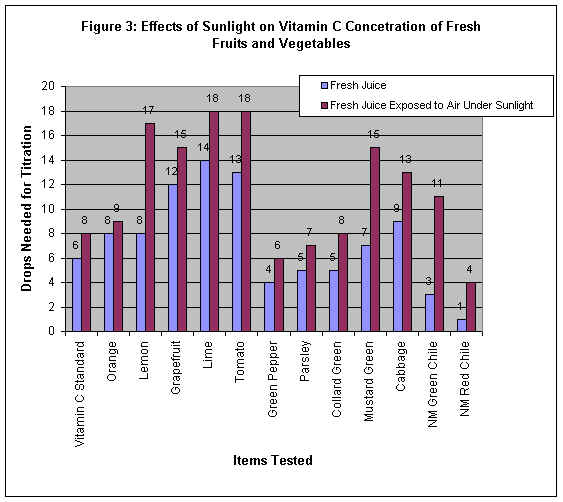
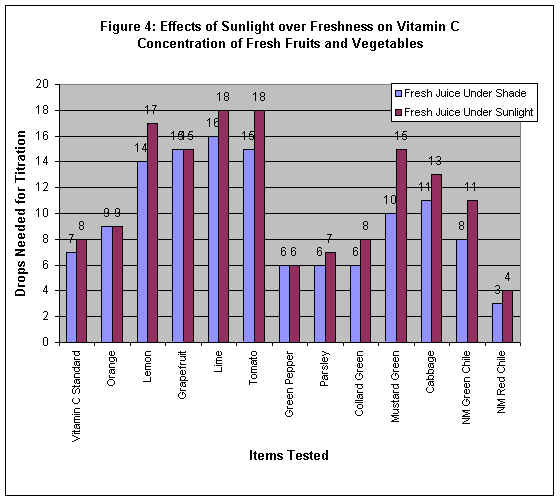
Interpreting the Data Obtained
Figure 1: Vitamin C Concentration of Fresh Fruits & Vegetables
The vitamin C standard needed six drops to change the royal blue corn starch-iodine
vitamin C indicator solution to clear. The more drops of a substance
containing vitamin C we needed to turn the solution clear, the weaker the
concentration of vitamin C in that substance. For example, orange and mustard
green were weaker in vitamin C concentration than the vitamin C tablet. Lime, grapefruit
and tomato had the weakest concentrations. Conversely, the less drops of a
substance containing vitamin C we needed to turn the solution clear, the stronger
the concentration of vitamin C in that substance. For example, parsley and collard
green were stronger in vitamin C concentration than the vitamin C tablet. Green pepper,
and New Mexico green and red chili had the strongest concentrations.
Figure 2: Effects of Freshness on Vitamin C Concentration of Fresh Fruits &
Vegetables
Fresh fruit or vegetable juice or vitamin C standard which had been exposed to the air
under shade for one hour lost vitamin C concentration in it compared to its fresh and non
exposed counterpart. This proved that vitamin C decomposes over time when exposed to the
air. The rate (or speed) of decomposition varied for different items tested. For example,
the highest rates of decomposition were found in lemon, New Mexico green chili and
grapefruit, whereas, the lowest rates of decomposition were found in vitamin C standard,
orange, parsley, and collard green among others.
Figure 3: Effects of Sunlight on Vitamin C Concentration of Fresh Fruits and Vegetables
Fresh fruit or vegetable juice or vitamin C standard which had been exposed to the air
under direct sunlight for one hour lost vitamin C concentration in it compared to its
fresh and non exposed counterpart. This proved that vitamin C decomposes over time when
exposed to the air, as well as to sunlight. The sunlight makes the decomposition rate
faster. For example, the concentrations of vitamin C in lemon and New Mexico green chili
were weaker when they were under sunlight compared to when they were under shade for one
hour.
Figure 4: Effects of Sunlight over Freshness on Vitamin C Concentration of Fresh Fruits
& Vegetables
Exposure to the air and exposure to sunlight are two factors that influence the rate of
decomposition of vitamin C in the items tested. For some items, sunlight had no effect
over exposure to the air, and for some other items the effect of sunlight over exposure to
the air varied. For example, on orange, grapefruit and green pepper sunlight had no effect
over the exposure to the air. Whereas, the highest effect of sunlight over exposure to the
air were found in lemon, tomato, and mustard green among others, and the lowest effect
were found in vitamin C standard, parsley, and New Mexico red chili among others.
Vitamin C & heat
Heated one cup of each item being tested, one at a time, to boiling and let it boil for
about 10 minutes. Let the item to cool down, and performed the titration procedure. No
amount of juice affected the royal blue corn starch-iodine solution. Heat had completely
destroyed the vitamin C concentration in all the items that were tested.
What did I learn?
- The royal blue colored corn starch-iodine solution does work as a vitamin
C indicator as suggested by the science experiment book.
- Orange and lemon are high in vitamin C concentration amongst the citrus
fruits tested in this experiment.
- New Mexico green and red chili and green pepper are high in vitamin C
concentration amongst the green vegetables tested in this experiment.
- This research experiment proved both my hypotheses to be true. The first
hypothesis is true because green pepper, New Mexico green and red chili, parsley, collard
green and mustard green had higher concentrations of vitamin C in them compared to orange,
lemon and other citrus fruits tested. The second hypothesis is true because we observed
that the concentration of vitamin C in the items tested either stayed the same or dropped
in the samples that were exposed to air, and sunlight for an hour.
- Vitamin C is unstable because it decomposed when exposed to the air. The
rate (speed) of decomposition varied between the fruits and vegetables tested.
- Vitamin C is unstable because it decomposed when exposed to sunlight. The
rate (speed) of decomposition varied between the fruits and vegetables tested.
- Heat (boiling for about ten minutes) had completely destroyed the vitamin
C concentration in all the items that were tested.
- In the future, it will be interesting to investigate the loss of vitamin
C from vegetables which have been cooked by various methods (various temperature
settings).
- Fresher the fruits and vegetables, higher the concentration of vitamin C
in them. Eating fresh fruits and vegetable salads will provide a lot of vitamin C to one
who catches a cold.
- New Mexico chilis are healthy, tasty, and provide a lot of vitamin C in
our diet.
- Based on the claim of late Dr. Linus Pauling in 1994 and the results
obtained from our experiment, people of New Mexico should have less heart diseases and
cancers compared to the rest of the nation because we New Mexicans love to eat our state
grown green and red chilis.
- Chemistry is a lot of fun!
Where did I gather the background information from?
Orii, E. and Orii, M., (1989) Simple Science Experiments with Water, Milwaukee,
WI: Gareth Stevens Children Books.
Penrose, G., (1990) Sensational Science Activities, New York: Simon and
Schuster.
VanCleave, J., (1989) Chemistry for Every Kid, New York: John Wiley and Sons.
Brisk, M., (1994) 1001 Ideas for Science Projects, Macmillan General Reference.
Tooley, P., (1975) Experiments in Applied Chemistry, London.
Podman, I. (1998) Eat Fruits and Vegetables, 5 a Day-for Better Health, National
Cancer Institute.
Gutnik, M., (1980) How to Do a Science project and Report, Franklin Watts, New
York.
|
